Performance-based risk sharing (PBRS) have become in importance in recent years. While most PBRS can be used for expensive pharmaceutical products, PBRS also provide been combined with medical device and diagnostic manufacturers. Device/diagnostic PBRS may differ from those of pharmaceuticals for several reasons. First, while health insurers bear the burden of coughing up for many pharmaceuticals, device and diagnostics are relatively more likely to be reimbursed by hospitals or health systems. Second, many device and diagnostics do not have randomized controlled trial evidence to support their launch, and thus there may be more uncertainty from the advantage of new devices and diagnostics. Third, devices and diagnostics typically are less expensive than pharmaceuticals.
How common PBRS arrangement for medical tool and diagnostics manufacturers? To answer this question, a recent paper by Chen and Carlson (2022) uses publicly available data on PBRS of these technologies. Specifically, the approach the authors took was the following:
We reviewed publicly available PBRSAs for diagnostics and devices, using the University of Washington Performance Based Risk Sharing (PBRS) Database. We augmented the review using PubMed, Google, and payer and industry websites, including the top 15 medical device and diagnostics companies by revenue and also the top ten medical health insurance companies by market price.. We further characterized arrangements based on our previously published taxonomy [Carlson 2010; Carlson 2022]. Three main categories were utilised: (1) coverage with evidence development (CED), only in research; (2) CED, only with research; and (3) performance-linked reimbursement (PLR).
Based on this approach, the authors discovered that:
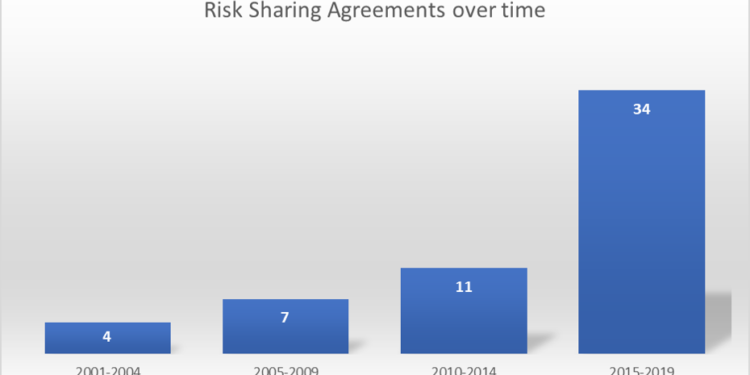
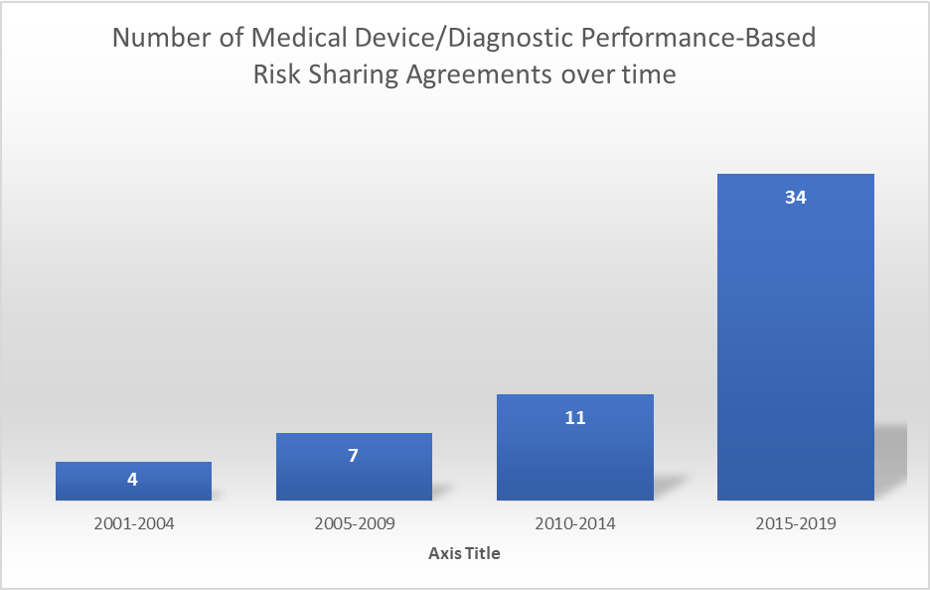
This review identified 52 performance-based arrangements which were initiated between 2001 and 2022. Of these, 23 (44.2%) were CED, only in research; 17 (32.7%) were PLR; and 12 (23.1%) were CED, just with research-
The utilization of PBRS has grown with time.
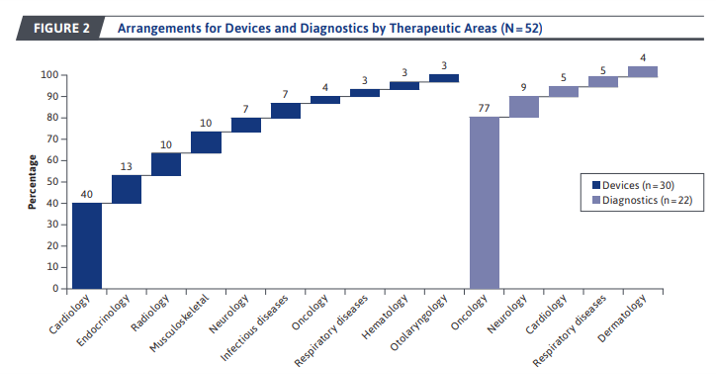
PBRS were most often employed for for cardiology devices and oncology diagnostics. Most of the PBRS arrangements in oncology were diagnostics used for precision medicine for example classifier assays, genomic profiling scores, or epigenetic testing. Examples of those precision medicine oncology tests include ConfirmMDx Epigenetic Molecular Assay, Prolaris, Decipher, and Oncotype DX Cancer of the prostate Assay. Insulin pump devices were one product that was commonly covered through PBRS arrangements.
How does the use of PBRS differ between public and private entities?
Most of the arrangements were area of the CED arrangements at the Centers for Medicare & Medicaid Services (CMS) or CMS contractors-of 52 arrangements, 34 (65.4%) were CEDs published around the CMS website and included 20 (58.8%) covered through national coverage determinations (NCDs) at CMS and 14 (41.2%) local coverage determination (LCDs) through the Medicare contractor Palmetto GBA. The rest of the arrangements (18, 34.6%) were identified from websites of non-public payers...
Of all arrangements, 5 (9.6%) were issued by private or commercial insurance companies, with 4 insulin pump devices for diabetes manufactured by Medtronic and another associated with UnitedHealthcare's arrangement to tie reimbursement of Genomic Health's Oncotype DX assay to a program of information collection in women considering adjuvant breast cancer therapy. Finally, arrangements being used by hospitals (11, 21.2%) and integrated health systems (2, 3.8%) covered a wide range of disease areas for medical devices.
Note that since the PBRS use publicly available sources, the findings of this study may be more skewed towards finding increased public utilization of PBRS since private entities might be less prepared to publicize using any sort of PBRS arrangement. The results may be skewed towards finding newer PBRS because more details could be available online for arrangements over the last years.
Despite these limitations, having a snapshot of the evolving landscape of performance-based risk sharing agreements among devices and diagnostics is a highly valuable contribution to the literature.
Source link










